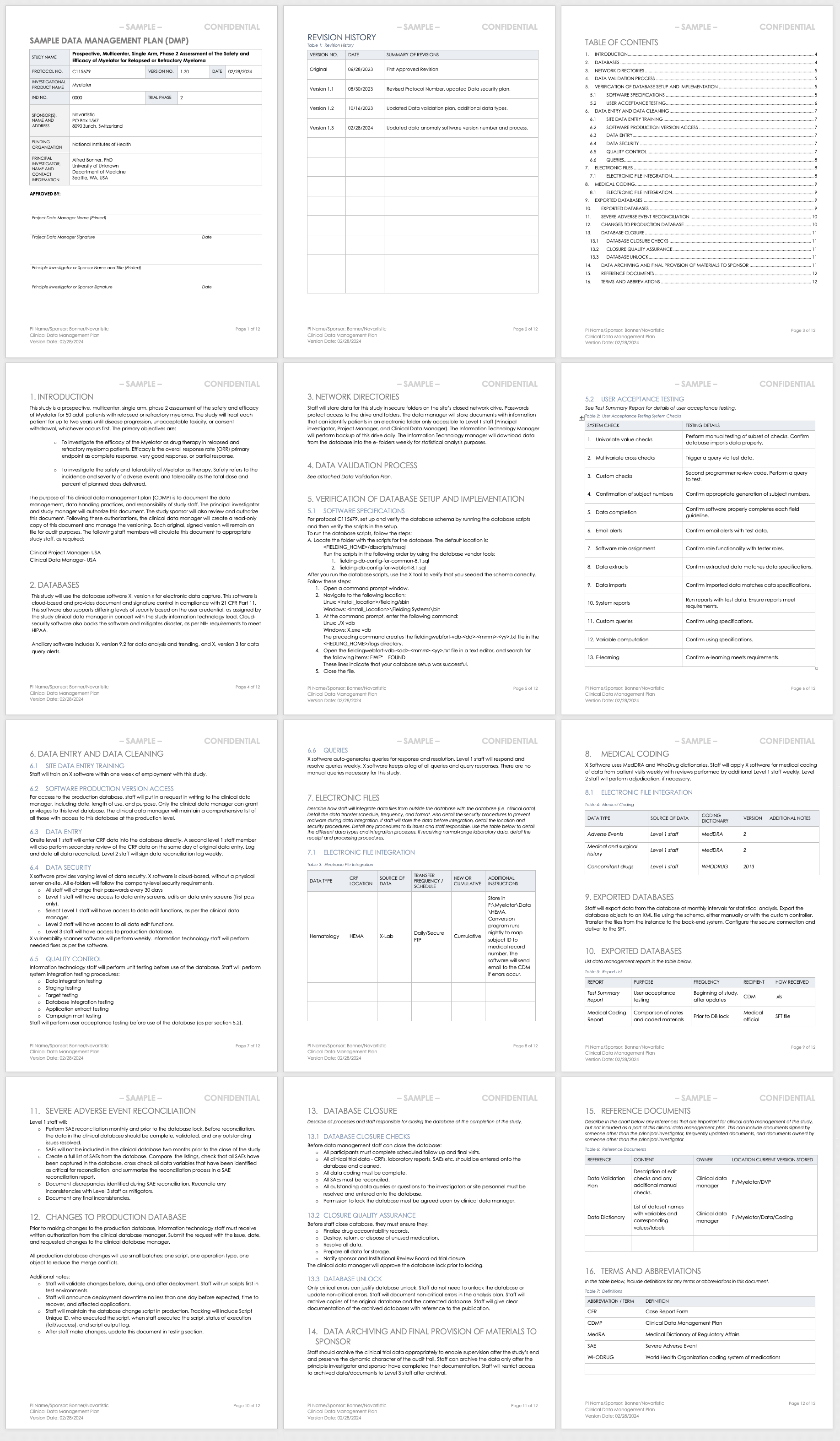
Schedule of enrollment, intervention, and assessment according to the... | Download Scientific Diagram

NHSBT/MRC Clinical Studies Unit Platelets for Neonatal Transfusion Study 2 (PlaNeT-2) A randomised controlled trial of platelet transfusion thresholds. - ppt download

David Cloutier Director, Research Center Management and Development Budgeting for Industry Sponsored Clinical Trials. - ppt download
PLOS Neglected Tropical Diseases: Therapeutics for Dengue: Recommendations for Design and Conduct of Early-Phase Clinical Trials

Dataset for Phase I randomized clinical trial for safety and tolerability of GET 73 in single and repeated ascending doses including preliminary pharmacokinetic parameters - ScienceDirect
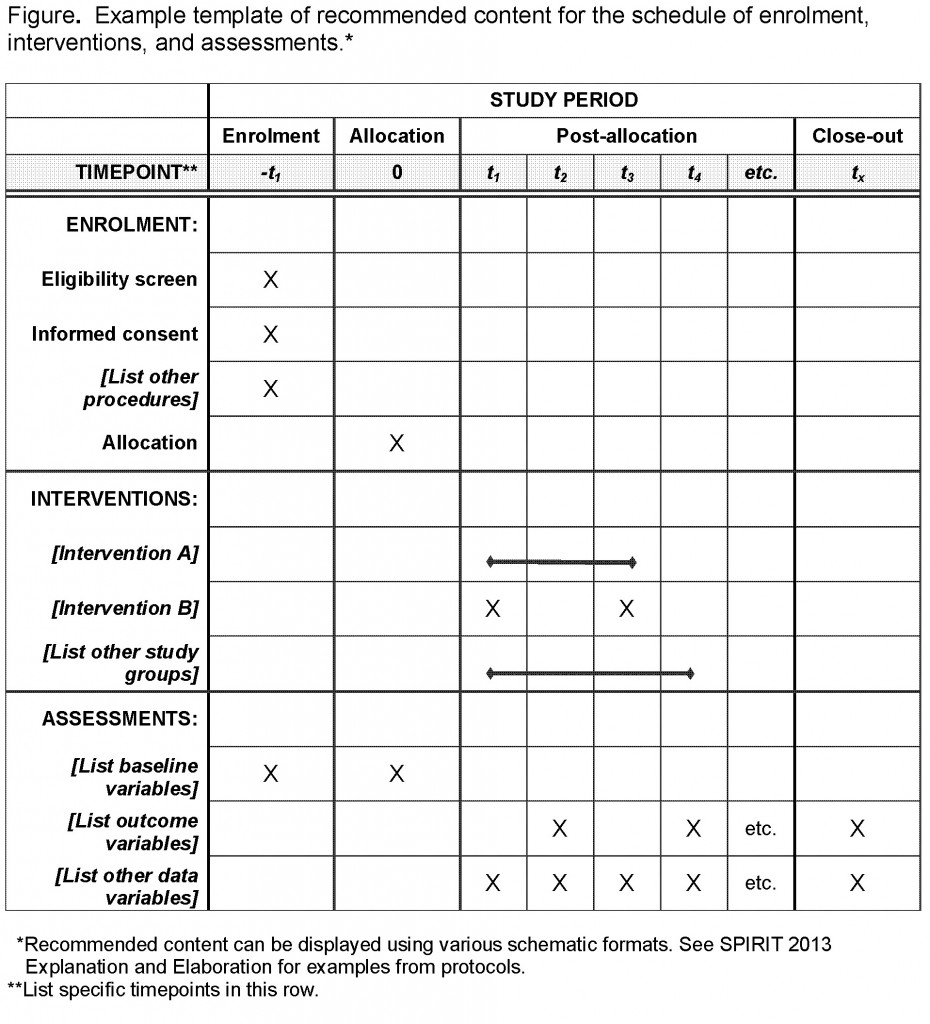
Continuation versus discontinuation of treatment for severe dementia: randomized, pragmatic, open-label, clinical trial to evaluate the efficacy of continuing drug treatment in patients with severe dementia (STOP-DEM) | BMC Geriatrics | Full
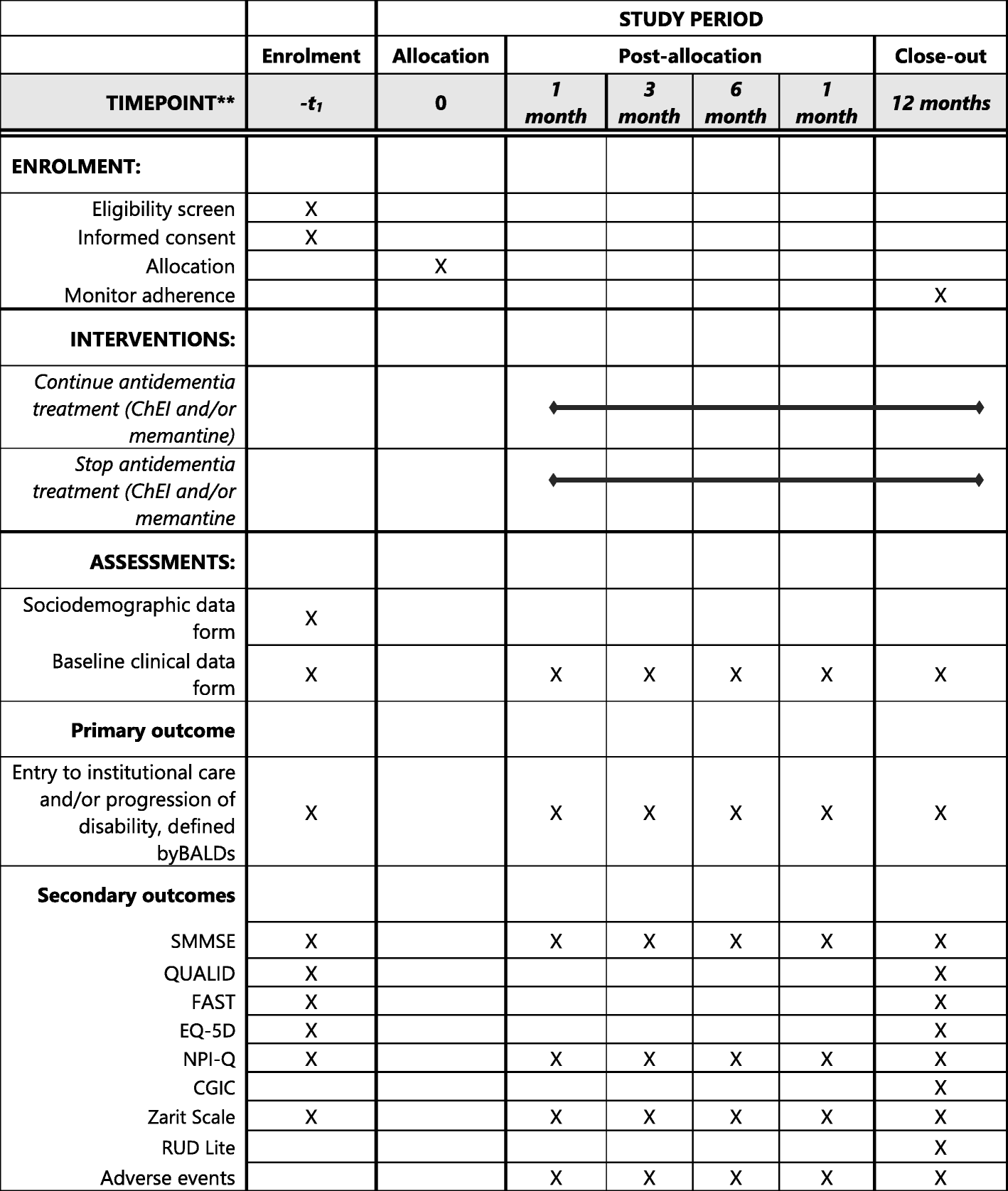
Effects of motor imagery training of Parkinson's disease: a protocol for a randomized clinical trial | Trials | Full Text

Schedule of enrolment, interventions and assessments in the ROSETTA... | Download Scientific Diagram

Protocol of the Definition for the Assessment of Time-to-event Endpoints in CANcer trials (DATECAN) project: Formal consensus method for the development of guidelines for standardised time-to-event endpoints' definitions in cancer clinical trials -