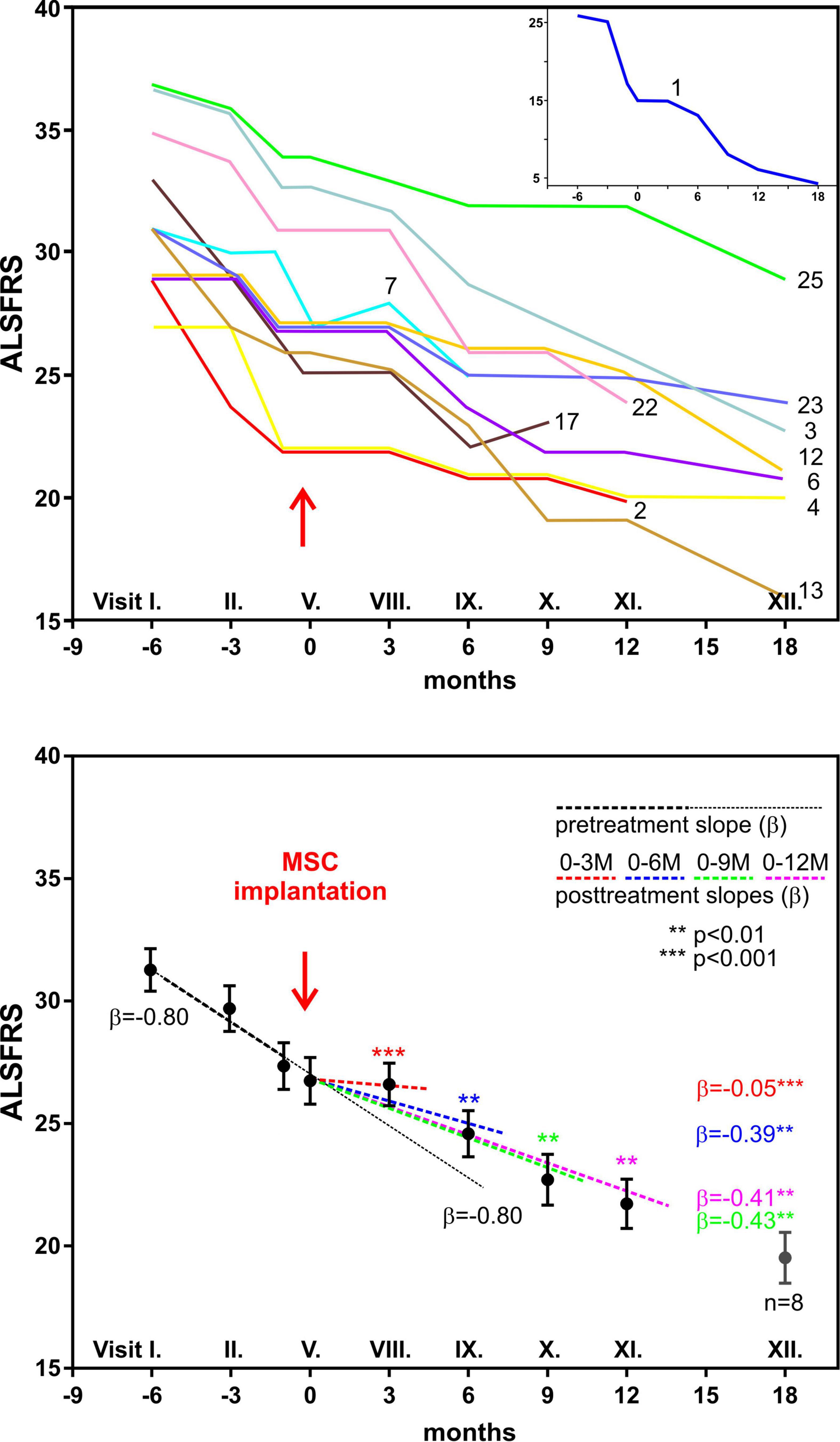
Mayo Clinic CELLTOP Clinical Trial: First Report From a Phase 1 Trial of Autologous Adipose Tissue–Derived Mesenchymal Stem Cells in the Treatment of Paralysis Due to Traumatic Spinal Cord Injury – REGENHEALTHSOLUTIONS (
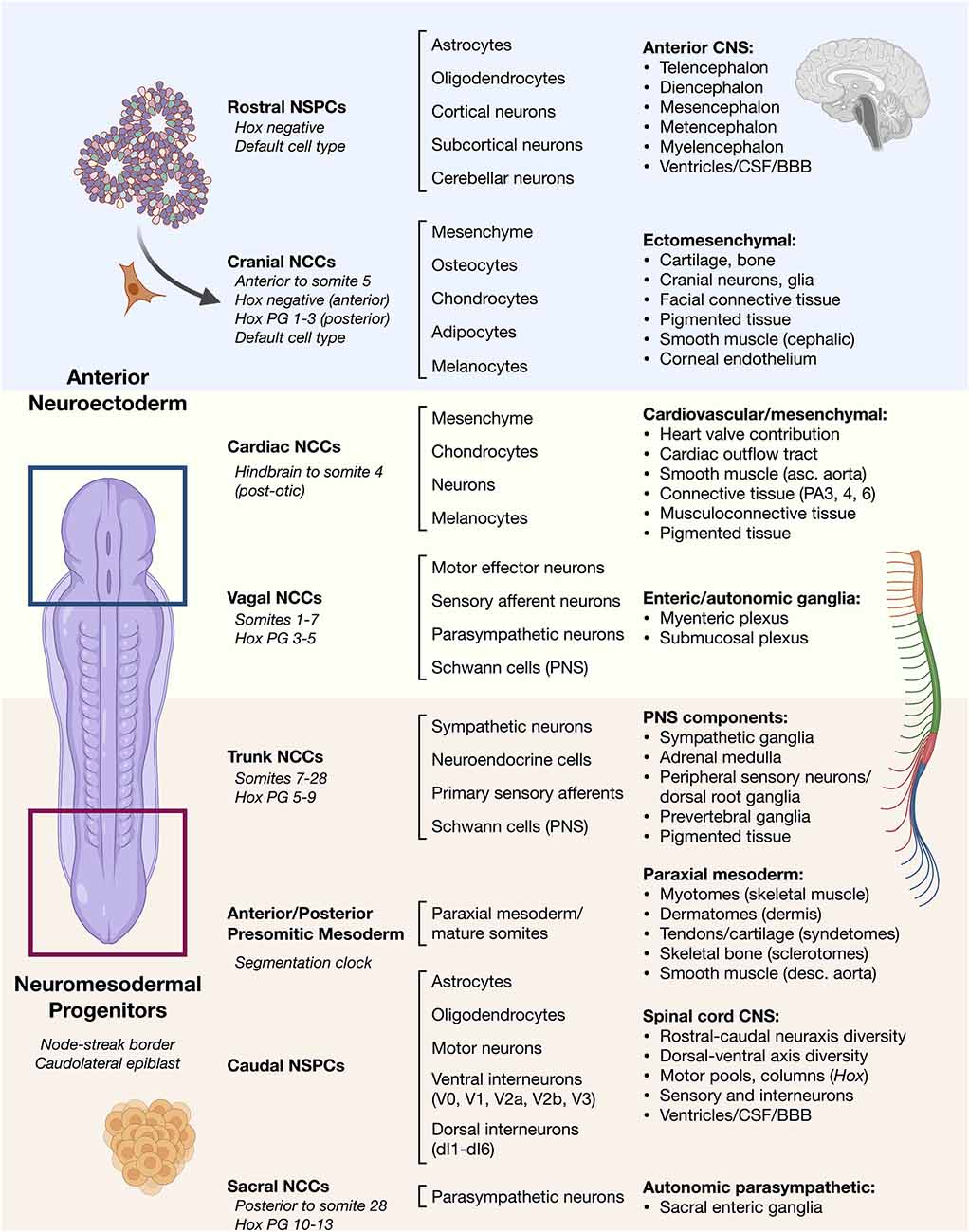
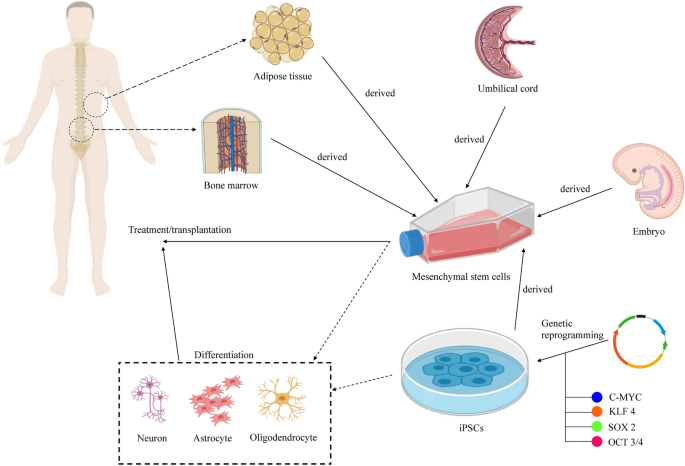
Frontiers | Stem Cell Neurodevelopmental Solutions for Restorative Treatments of the Human Trunk and Spine | Cellular Neuroscience
CELLTOP Clinical Trial: First Report From a Phase 1 Trial of Autologous Adipose Tissue-Derived Mesenchymal Stem Cells in the Tre

CELLTOP: A Phase I Clinical Trial of Autologous Adipose Derived Mesenchymal Stem Cells in the Treatment of Paralysis due to Traumatic Spinal Cord Injury | Regenerative Medicine Minnesota
CELLTOP Clinical Trial: First Report From a Phase I Trial of Autologous Adipose Tissue--Derived Mesenchymal Stem Cells in the Treatment of Paralysis Due to Traumatic Spinal Cord Injury. - Document - Gale
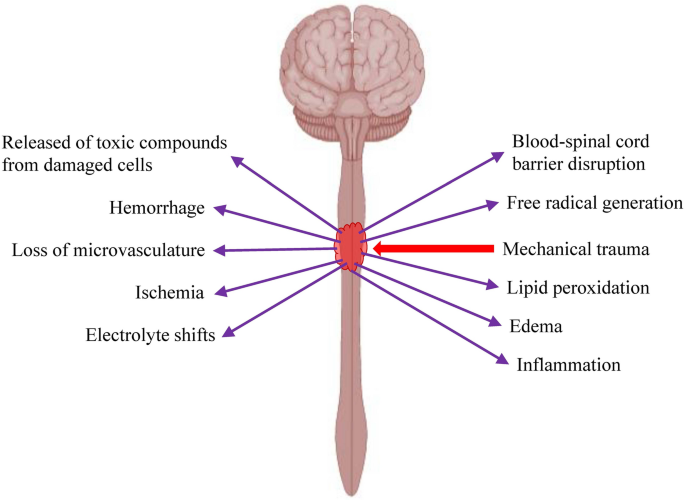
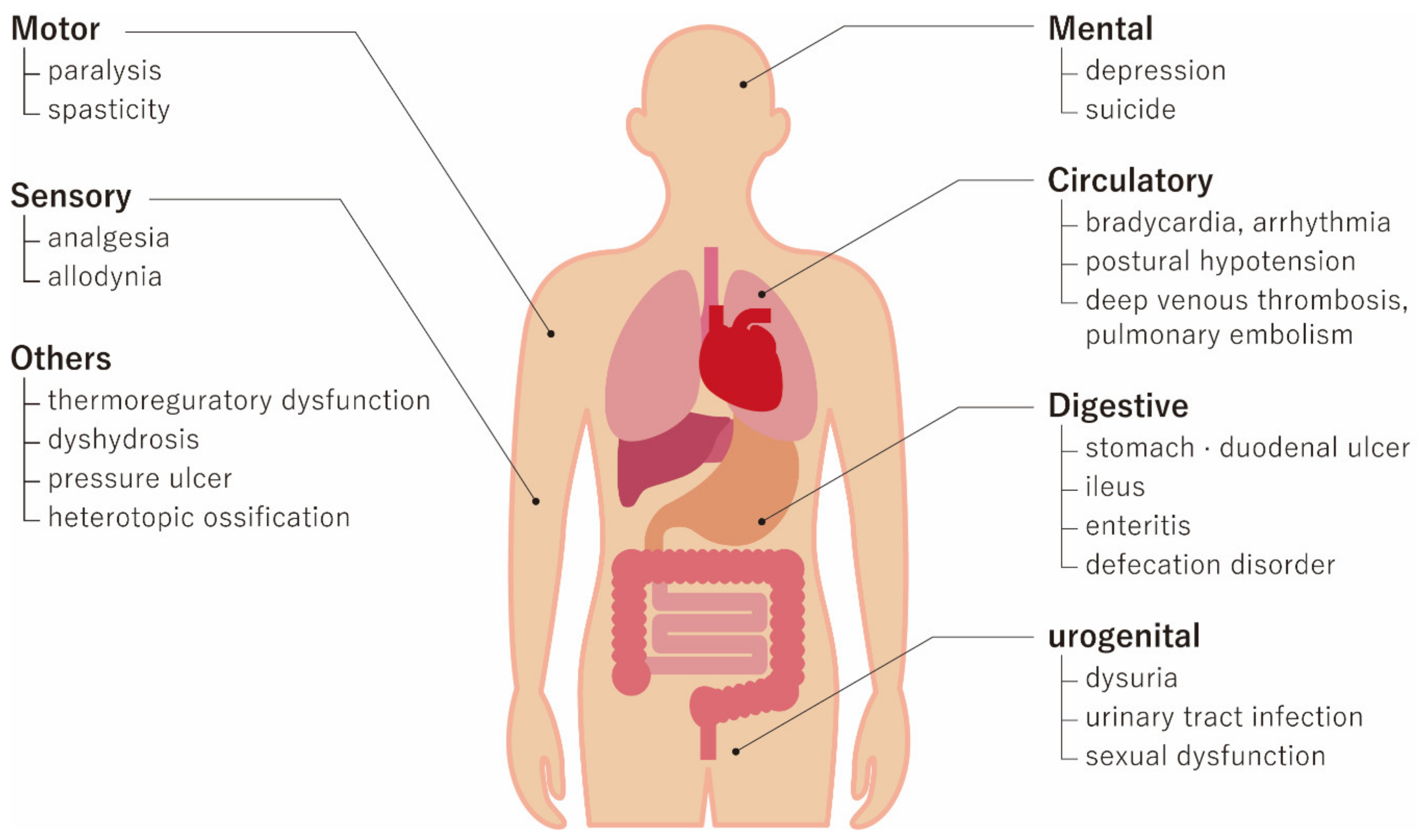
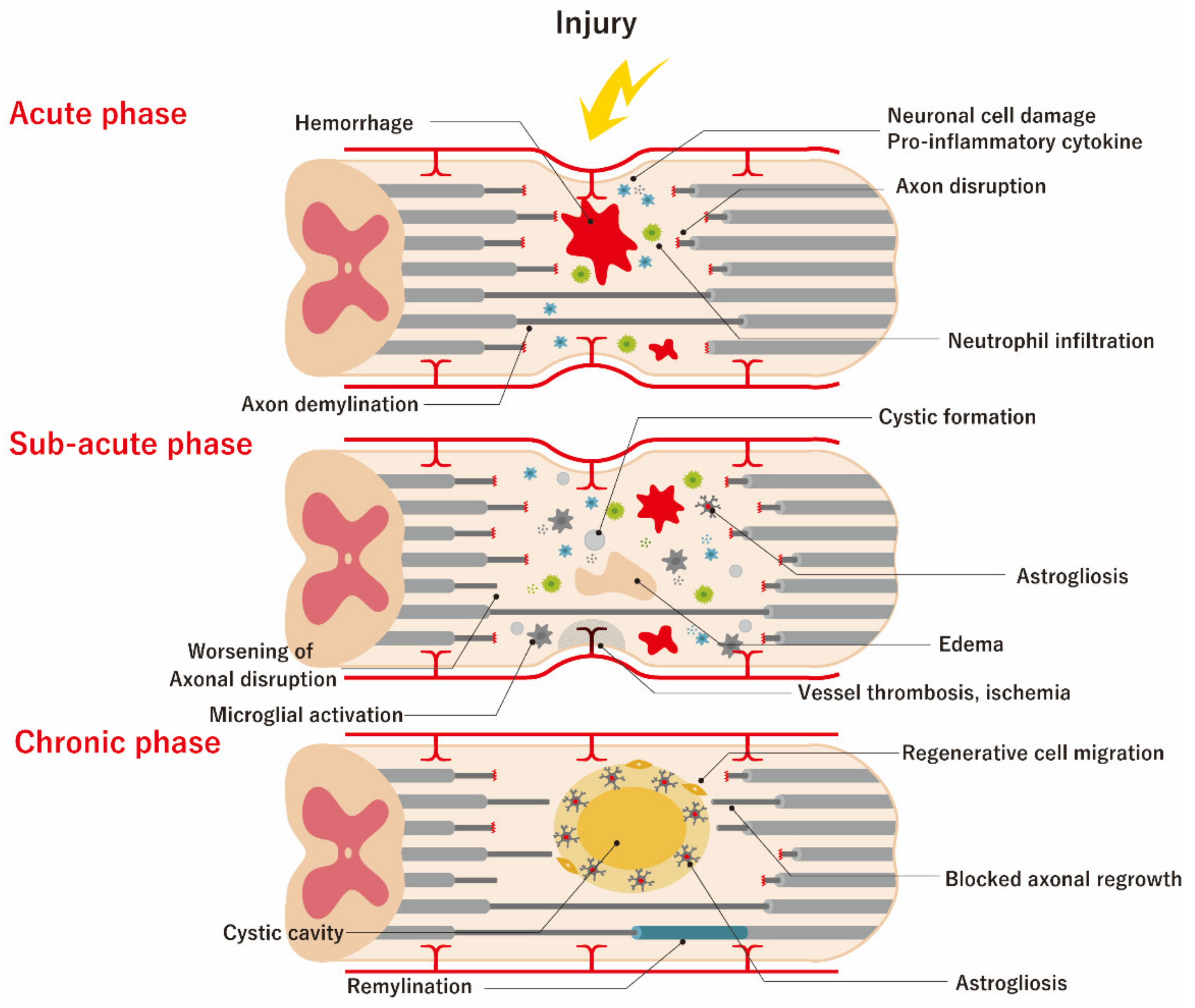
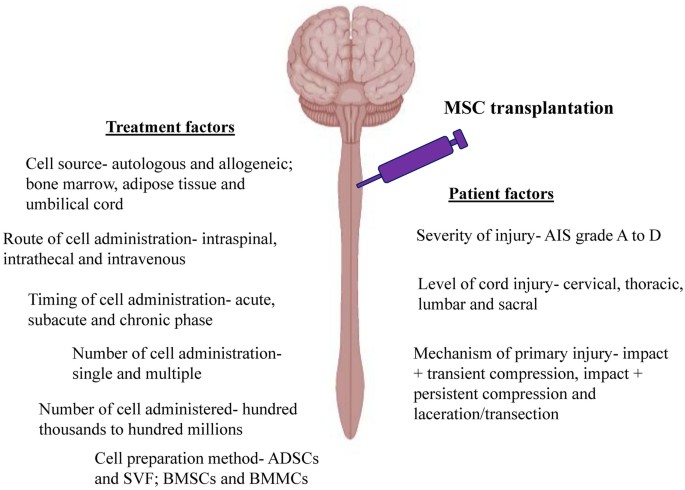
Clinical Trials Using Mesenchymal Stem Cells for Spinal Cord Injury: Challenges in Generating Evidence

CELLTOP Clinical Trial: First Report From a Phase 1 Trial of Autologous Adipose Tissue–Derived Mesenchymal Stem Cells in the Treatment of Paralysis Due to Traumatic Spinal Cord Injury - Mayo Clinic Proceedings

CELLTOP: A Phase I Clinical Trial of Autologous Adipose Derived Mesenchymal Stem Cells in the Treatment of Paralysis due to Traumatic Spinal Cord Injury | Regenerative Medicine Minnesota

CELLTOP: A Phase I Clinical Trial of Autologous Adipose Derived Mesenchymal Stem Cells in the Treatment of Paralysis due to Traumatic Spinal Cord Injury | Regenerative Medicine Minnesota

CELLTOP Clinical Trial: First Report From a Phase 1 Trial of Autologous Adipose Tissue–Derived Mesenchymal Stem Cells in the Treatment of Paralysis Due to Traumatic Spinal Cord Injury - Mayo Clinic Proceedings
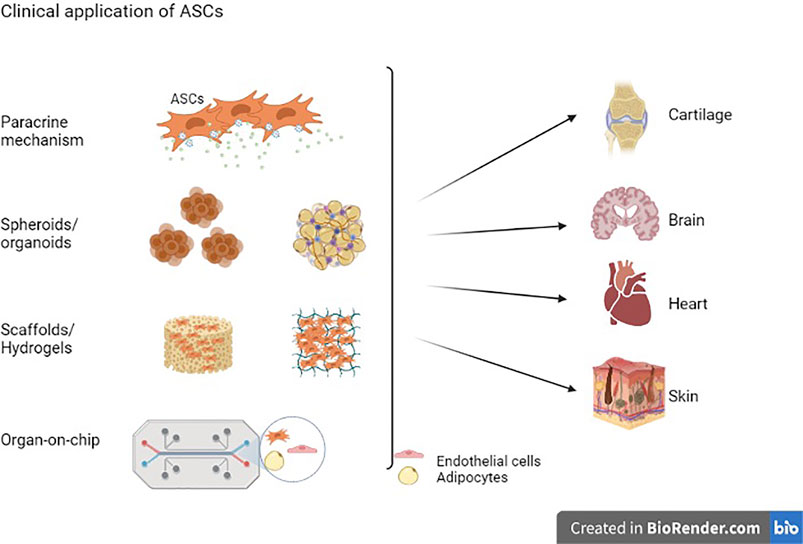
Frontiers | Adipose Stem Cells in Regenerative Medicine: Looking Forward | Bioengineering and Biotechnology

CELLTOP Clinical Trial: First Report From a Phase 1 Trial of Autologous Adipose Tissue–Derived Mesenchymal Stem Cells in the Treatment of Paralysis Due to Traumatic Spinal Cord Injury - ScienceDirect

CELLTOP Clinical Trial: First Report From a Phase 1 Trial of Autologous Adipose Tissue–Derived Mesenchymal Stem Cells in the Treatment of Paralysis Due to Traumatic Spinal Cord Injury - Mayo Clinic Proceedings
CELLTOP Clinical Trial: First Report From a Phase I Trial of Autologous Adipose Tissue--Derived Mesenchymal Stem Cells in the Treatment of Paralysis Due to Traumatic Spinal Cord Injury. - Document - Gale

Mayo Clinic CELLTOP Clinical Trial: First Report From a Phase 1 Trial of Autologous Adipose Tissue–Derived Mesenchymal Stem Cells in the Treatment of Paralysis Due to Traumatic Spinal Cord Injury – REGENHEALTHSOLUTIONS (
Clinical Trials Using Mesenchymal Stem Cells for Spinal Cord Injury: Challenges in Generating Evidence