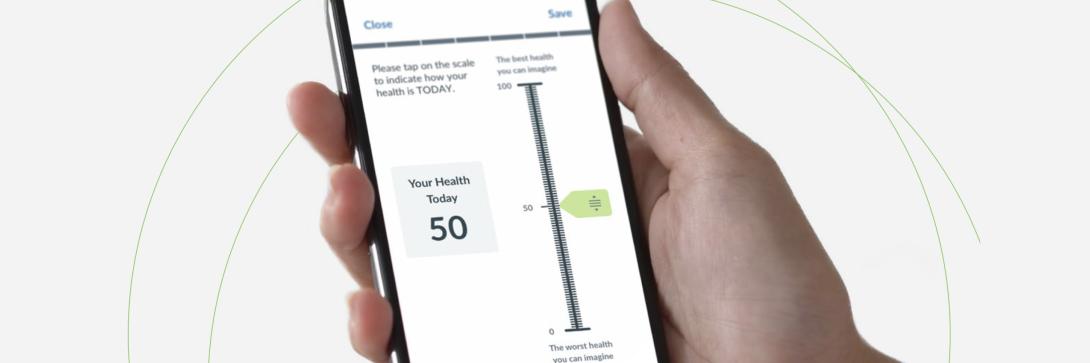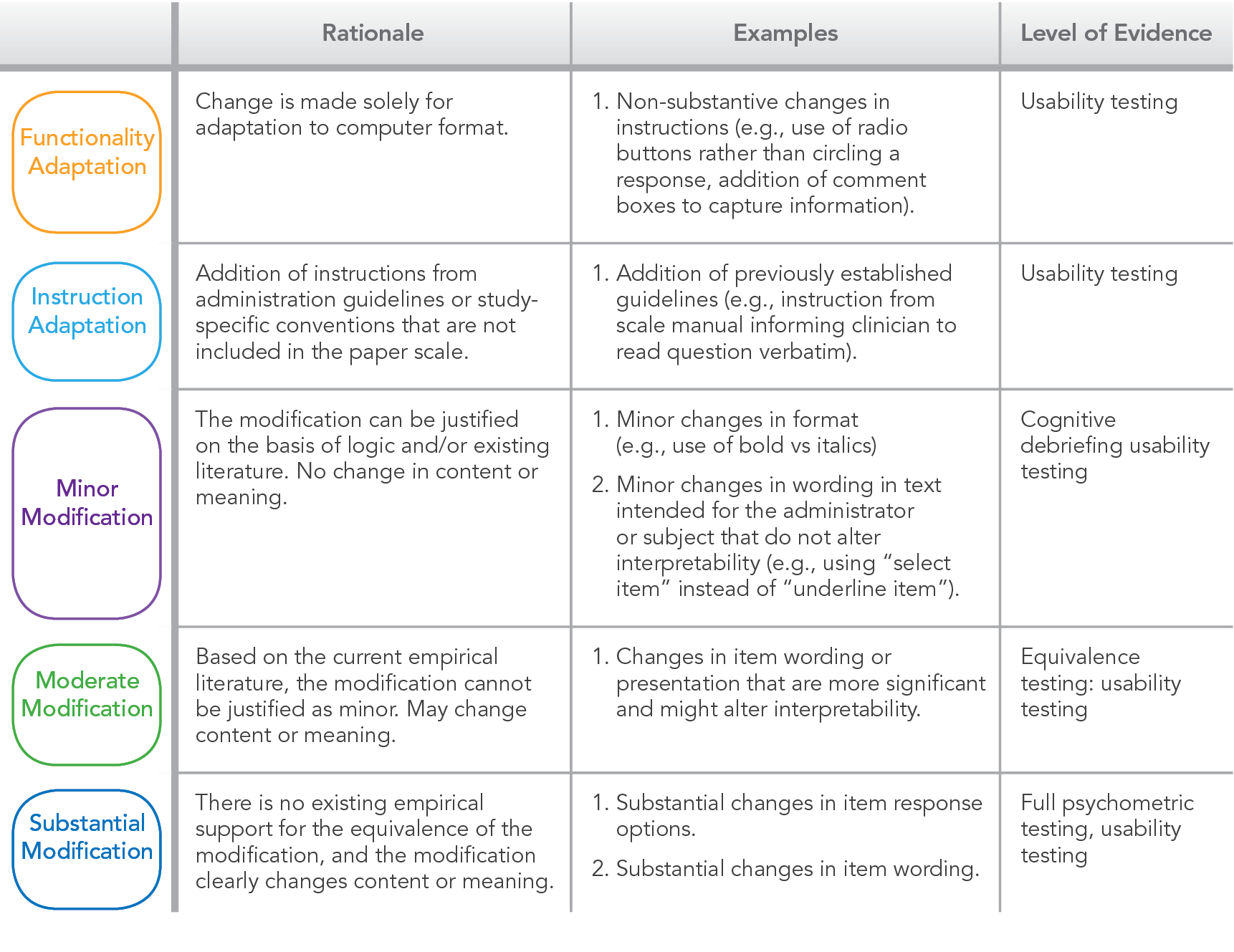
White Paper: Surprises You Don't Want When Adopting eCOAs for Use in Clinical Trials: Cautions for Decision Making and Planning - Evidera
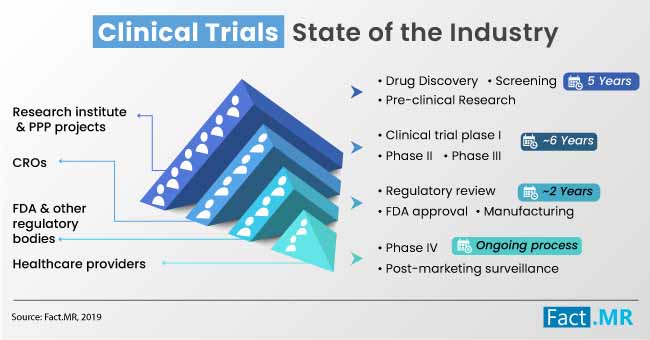
Global eCOA, eSource & Clinical Trials Market: Emerging Economies Expected to Influence Growth until 2029 - PharmiWeb.com
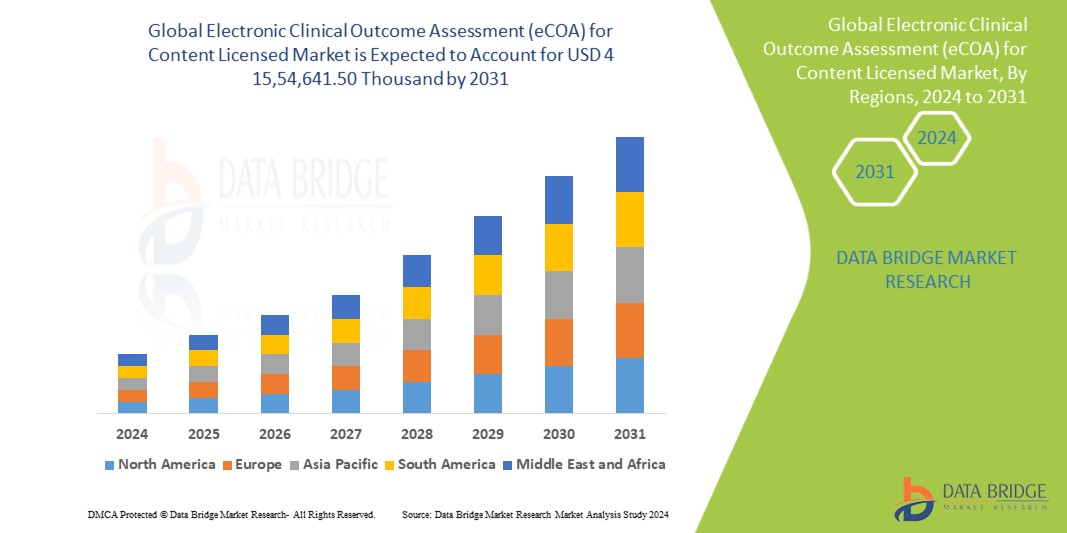
Electronic Clinical Outcome Assessment (eCOA) for Content Licensed Market – Global Industry Trends and Forecast to 2028 | Data Bridge Market Research